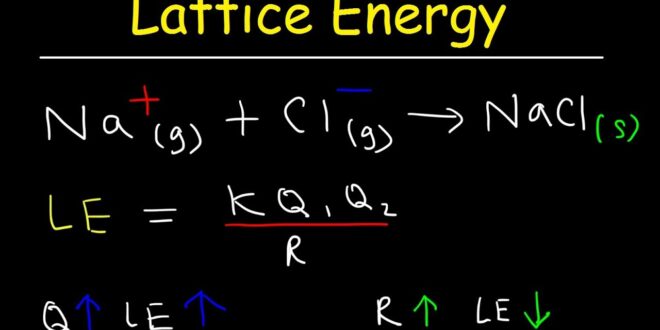
Lattice energy trend is a trend that is frequently seen in the periodic table. It is known as the energy released when ions, who are opposite, are changed into a solid-state from gaseous state. They can be calculated using Coulomb`s law. This energy is decreased when individuals are tied down to a certain group from the periodic table.
The reason is that the atomic radius will increase during those downward movements. Plus, it is important to point out that lattice energy will increase together will an increase in the charge magnitude. That goes both for negative and positive changes. More specifically, the lattice energy is needed when energy converts crystals into molecules or atoms.
This is the older definition. Today, there is a new one. The new version says that lattice energy is essential for the transformation of atoms, molecules, and ions into crystals. Basically, these definitions are pretty similar. It is important to say that the lattice energy is much greater in crystals than is solids. So, one of the examples is that sodium chlorine consists of bigger lattice energy than sugar, so to say.
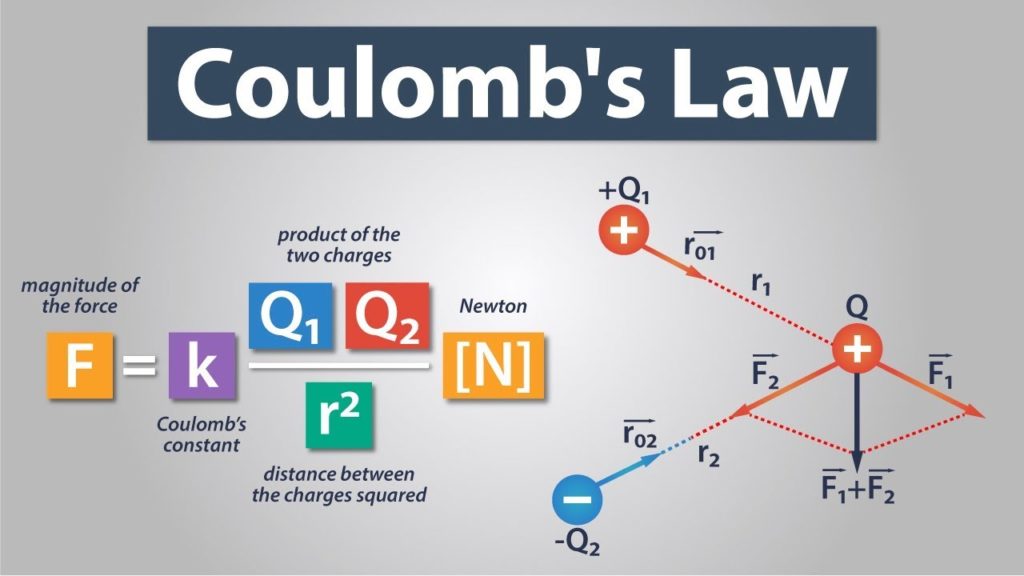
Finding an exact amount of lattice energy`s magnitude is not possible, because it is not possible to create atoms or ions. As we said, the lattice energy trend is something that repeats itself every now and then. Also, we would like to say that the lattice energy is also known as lattice enthalpy. This energy cannot be measured, but it can be calculated by using estimated with Born-Haber cycle or electrostatics.
Now, we are going to present you with factors that have a certain influence on lattice energy.
Lattice Energy and Factors
Two factors can have an impact on the lattice energy trend.
- Ionic Radius is a measurement of ion`s size. Lattice energy is higher in the compounds that consist of small ions because they are packed together close.
- Ionic Change shows that ions that have greater charge have a higher amount of lattice energy.
 Hi Boox Popular Magazine 2024
Hi Boox Popular Magazine 2024